
Foam gushes out when you shake a bottle or a can of beer and open it.
This occurs because the carbon dioxide that had dissolved in the beer gasifies and escapes. In the case of carbon dioxide, it reaches the limit of dissolution, or saturation, at about 0.2% in water and 0.17% in beer, at a temperature of 15oC and 1 atmosphere pressure. In reality, however, beer contains nearly 0.5% carbon dioxide. The state is referred to as oversaturation, which is highly unstable, and the gas contained in the liquid tends to escape easily. However, beer remains relatively stable even when it contains an oversaturated amount of carbon dioxide. The reason is believed to be that colloidal substances contained in the beer adsorb the carbon dioxide. When the beer is shaken or given a shock, the stable state is broken and the gas gushes out.
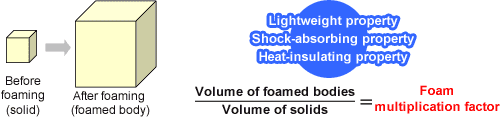
It is foamed plastics that make use of these phenomena to encapsulate air into them. By encapsulating the air into plastics, the weight can be reduced compared to plastics of the same volume. The air contained also provides heat-insulating and cushioning properties.
Foaming refers to
The volume is expanded using air bubbles. This enables a rather lightweight material to be created even when it occupies a large volume. Also, the air layer adds heat-insulating and shock-absorbing (cushioning) properties.
Independent air bubbles
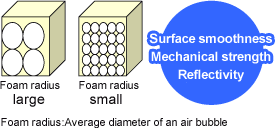 Unchained air bubbles that contain the air are referred to as independent air bubbles. Most of Furukawa’s products are made of materials that have independent air bubbles.
Unchained air bubbles that contain the air are referred to as independent air bubbles. Most of Furukawa’s products are made of materials that have independent air bubbles.
Continuous air bubbles
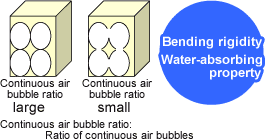 Chained air bubbles are referred to as continuous air bubbles.
Chained air bubbles are referred to as continuous air bubbles.